Data Management
We aim to answer questions such as: How can I meet ethical and legal standards while respecting the epistemological and methodological codes of my discipline? How to respond to the growing demand for data openness without violating the new data protection rules? How do I store my data securely? Can I share everything? What will happen to my data after I retire?
You will find here useful resources that will help you to develop a strategy adapted to your needs, be it for data management planning or its implementation throughout a research project. Much more than an administrative procedure, data management is an opportunity to reflect on fundamental issues related to the production and processing of data, but also on the potential of data beyond the research projects for which they were initially collected.
Data management practices throughout the data life cycle
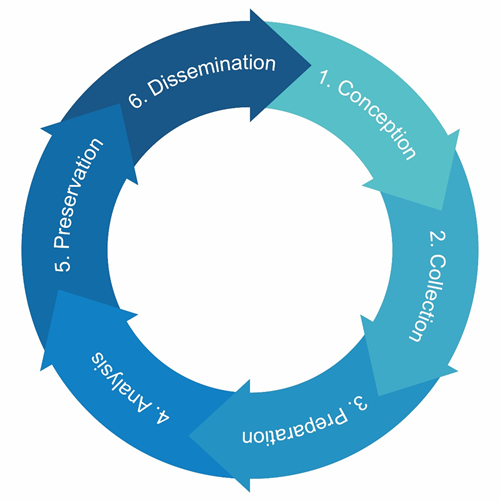
In Switzerland
In Europe
Worldwide
FORS Guide N°3: Ethics in the era of open research data: some points of reference
FORS Guide N°5: The informed consent as legal and ethical basis of research data production
We also draw your attention to the fact that more and more stakeholders are demanding an ethical compliance certificate from researchers: funders, publishers, research fields, and governments. At the Swiss level, research that falls within the scope of the Federal Act on Research involving Human Beings (HRA) must be submitted to the competent cantonal commission. For projects not subject to the HRA, some universities have specialized commissions.
FORS Guide N°7 : How to draft a DMP from the perspective of the social sciences, using the SNSF template
Currently available FORS guides can be found here.
Anonymization
Informed consent
User contracts
• Implementation of DMPs (data management plans)
• Compliance to ethical and legal standards
• Documentation practices
• Informed consent
• Anonymisation methods
• Data security and storage
• File management
• Archiving and sharing
We offer workshops and can also support you with individual consulting on the management of your qualitative or quantitative data. If you need our support, contact us.







 Bâtiment Géopolis,
Bâtiment Géopolis, +41 (0)21 692 37 30
+41 (0)21 692 37 30

